How Does Concentration Affect Cell Potential
What affects a galvanic cell. The concentration effect in an electrochemical cell is described by The Nernst Equation.
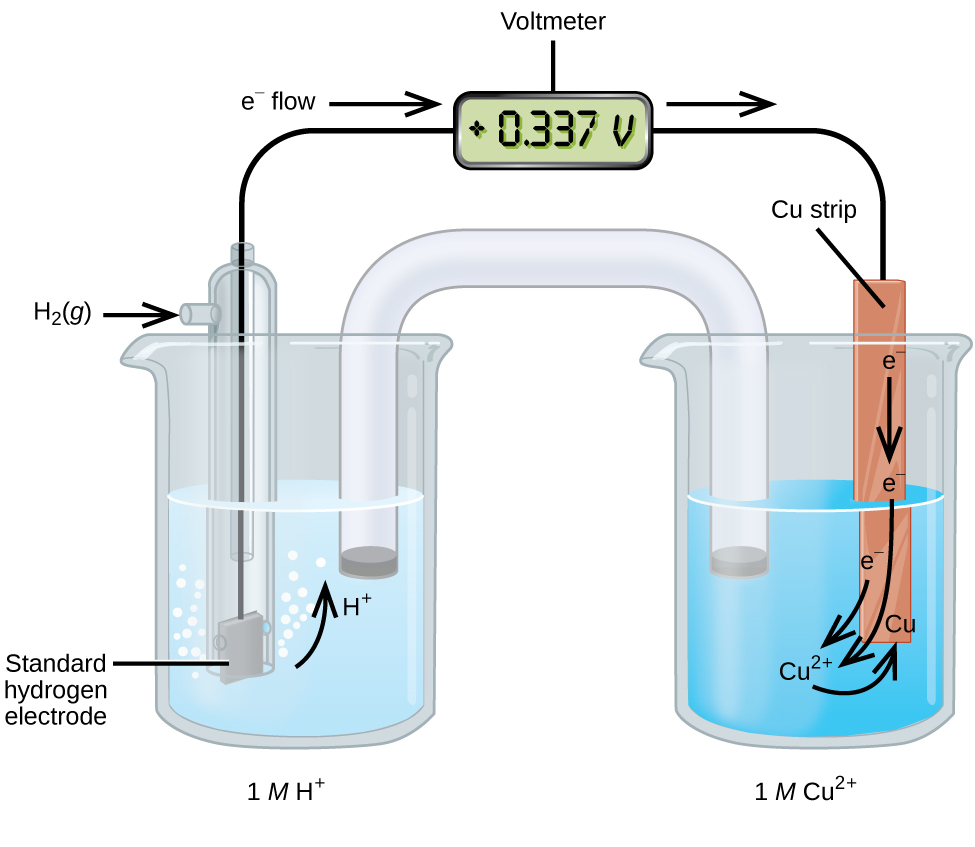
17 3 Standard Reduction Potentials Chemistry
Because ΔG 0 at equilibrium the.
. Standard electrode potentials are always specified as the voltage potential under standard conditions 25C 100 mol L-1 as the voltage is dependent upon both temperature and electrolyte concentration. Therefore an increase in concentration must cause the equilibrium to move to the left hand. The Nernst equation allows us to determine the spontaneous direction of any redox reaction under any reaction conditions from values of the relevant standard electrode potentials.
Here the effect of temperature and concentration on the voltage of a Daniell cell is quantified. How does concentration affect the cell potential. The relationship between cell potential and ion concentration is given by the Nernst equation.
Increasing extracellular K increases the positive charge outside the cell. How do temperature and concentration affect Ecell of a half cell An ongoing discussion beginning back in 2003. To optimize the voltage in a GalvanicVoltaic Cell the concentration of the Reducing Agent in the Anodic Side of the system oxidation rxn should be low and the concentration of the Oxidizing Agent ion in the Cathodic side of the system reduction rxn should be high.
Of course a requirement is that the the reaction quotient remains at 1 because as soon as it differs the Δ E no longer represents the standard electrical potential of the directly cell and so it must be. Because the concentration in only one side of the cell has changed the concentration gradient across both sides of the cell becomes steeper. In the 20th century German chemist Walther Nernst proposed a mathematical model to determine the effect of reactant concentration on electrochemical cell potential.
Zns Cu2 aq Zn2 aq Cus E0 cell 11V. The Nernst equation tells us that a half-cell potential will change by 59 millivolts per 10-fold change in the concentration of a substance involved in a one-electron ox. How does concentration affect the cell potential.
A There is this nifty little mathematical relationship called the Nernst equation that allows us to deal with the cell potential of nonstandard solutio. This is the emf of the cell when operating under standard conditions Ie 1 Atmosphere 298K and unit concentration. The further the equilibrium is to the right the more negative the electrode potential.
As the system discharges the concentration of Reducing Agent ion will increase due. Changing the concentration of one solution in the cell will increase the voltage potential of the cell because you are putting the system further out of equilibrium. This is where the temperature dependence comes from.
Hi its me again. Δ G Δ H T Δ S. For instance as potassium levels increase in the extracellular space the magnitude of the concentration gradient for potassium across the myocyte diminishes thus decreasing the resting membrane potential that is 90 mV to 80 mV.
To determine the effect of concentration on cell potential using the Nrest equation. Also know how does concentration affect cell potential. This decreases the difference between the inside and outside of the cell.
The Effect of Temperature and Concentration on Galvanic Cells. The electrode potential depends on the equilibrium for example M s M2 aq 2e. Changing the concentration of one solution in the cell will increase the voltage potential of the cell because you are putting the system further out of equilibrium.
I The Nernst Equation Reported cell potentials are typically measured under standard conditions. I carried out some experiments where I varied the concentration of a copper half cell and compared the potential difference against a silver half cell. I will show how this works by this example.
Im a bit confused about why in 1c if the air pressure is lower and there is a lower concentration of oxygen why the cell potential decreasesIt checks out fine with the Nernst Equation but conceptually voltage is the driving force or energy of the current. We have the Nerst Equation. According to faradays law how does the concentration of an electrolyte affect the amount of the substance deposited at an electrode during an electrochemical reaction please help me tanks in.
Also using Le Chateliers principle an increase in concentration of Mn2 causes equilibrium to shift as the reaction goes toward the products producing a higher positive voltage. In the late 19th century Josiah Willard Gibbs formulated a theory to predict whether a chemical reaction would be spontaneous based on free energy. An equal concentration of the solutions will result a cell potential equal to the standard because the logarithm of the reaction quotient will be 0.
ΔG ΔGo RT ln. Like how the standard reduction potential isnt based on moles of reactant. Any good chemistry text will have a section on this topic.
It has been determined that cell potentials are related to concentrations of reactants and products and to. Why does changing the extracellular K concentration have more of an effect on the membrane potential than changing the extracellular Na concentration. Δ E Δ S n F T Δ H n F.
By the equilibrium law. As the reaction shifts toward the right Ecathode increases which therefore increases Ecell since Ecell Ecathode - Eanode. Kc M2 M s cannot appear in the equilibrium law equation as its not in the same state.
If we alter these conditions in this case. View the full answer. How does potassium affect the resting membrane potential.
Factors such as the metals constituting the electrodes play a large role in determining the voltage potential of a galvanic cell however other variables such as concentration and temperature do affect the voltage produced. Effect of Concentration Changes on Cell Potential 1. How does K affect membrane potential.

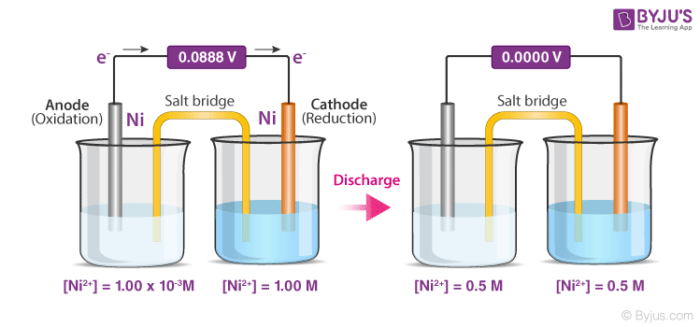
Concentration Cells Cell Potential Calculations Electrochemistry Youtube

Electrolyte Concentration An Overview Sciencedirect Topics

Chemistry Notes Chemistry Pdf Electrochemistry And Galvanic Cells Chemistry Notes Electrochemistry Teaching Chemistry

Factors Affecting Chemical Equilibrium Equilibrium Chemical Chemical Reactions

Question Video Calculating The Standard Cell Potential For A Gold Nickel Cell Nagwa

Pin By Ana Zivkovik On Chemistry Chemistry Notes Chemistry Electrochemistry
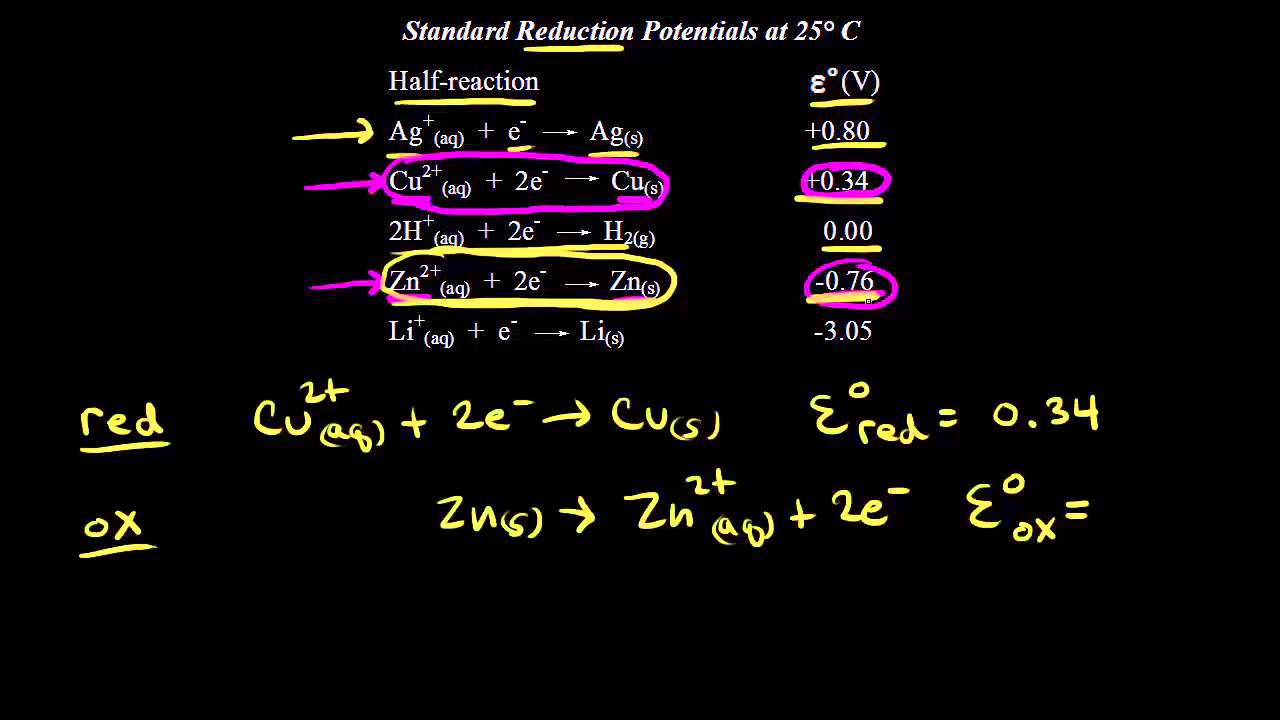
Standard Reduction Potentials Video Khan Academy

Concentration Cell Definition Details Types And Components With Examples
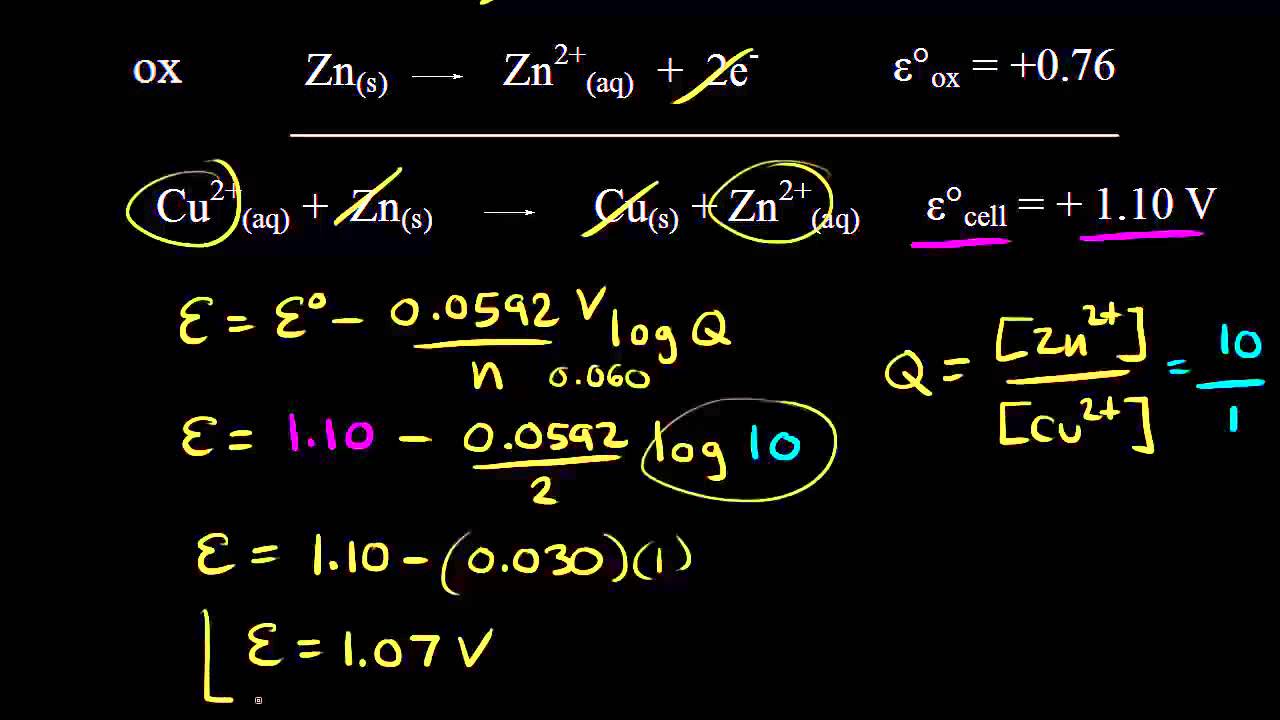
Using The Nernst Equation Video Khan Academy

Ion Across The Plasma Membrane Current I Will Cause K Leak Out Leading To A Hyperpolarization Negative Physiology Extracellular Fluid Cell Membrane
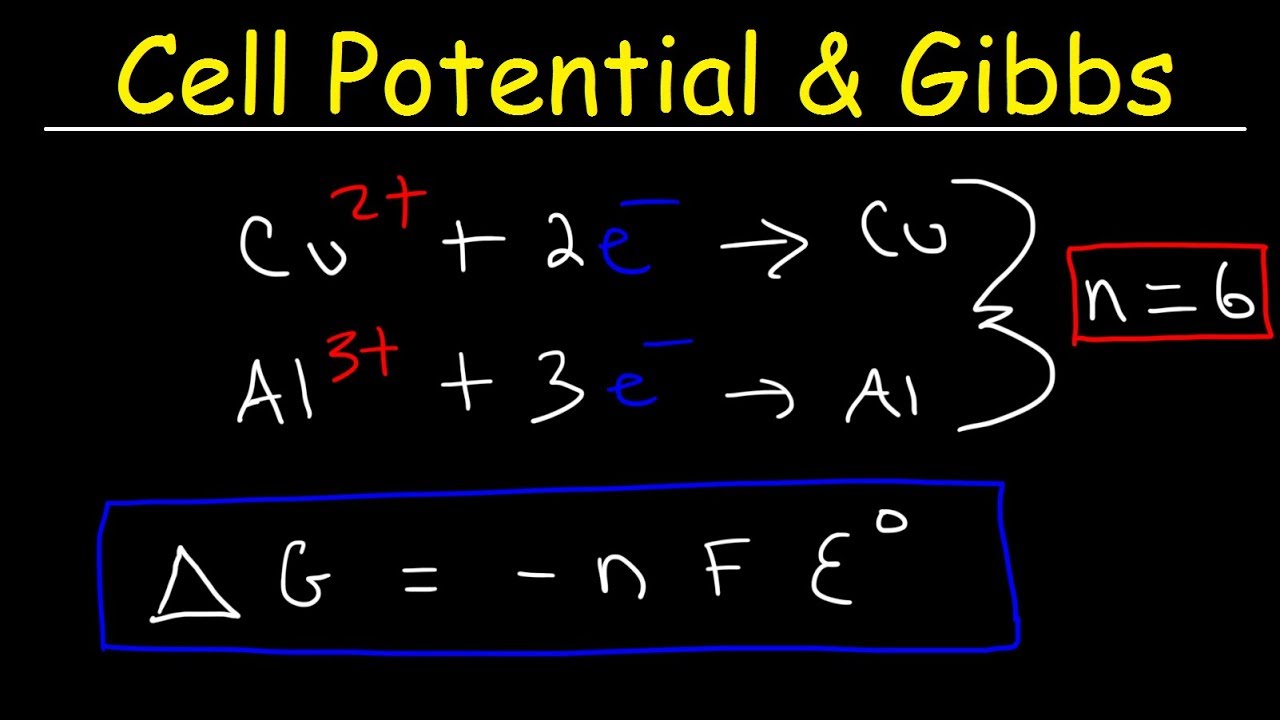
Concentration Cells Cell Potential Calculations Electrochemistry Youtube

Standard Electrode Potential Definition Significance Spontaneity Of Reactions
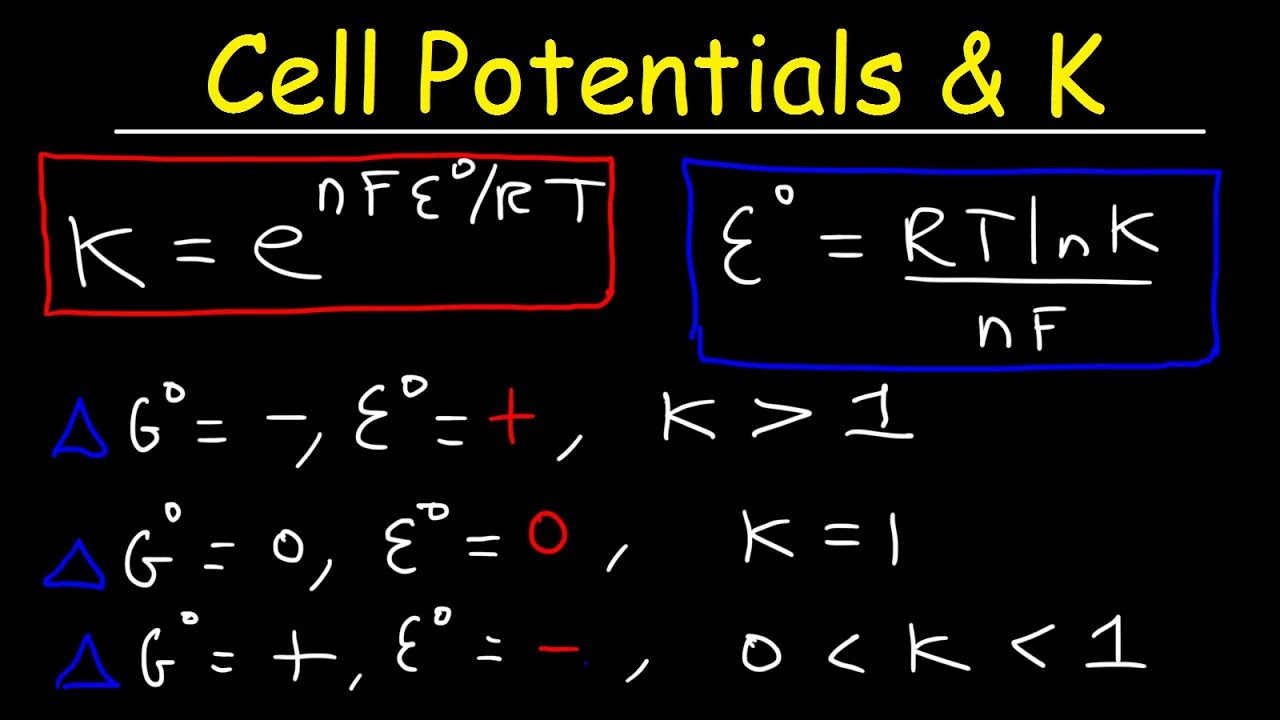
Concentration Cells Cell Potential Calculations Electrochemistry Youtube
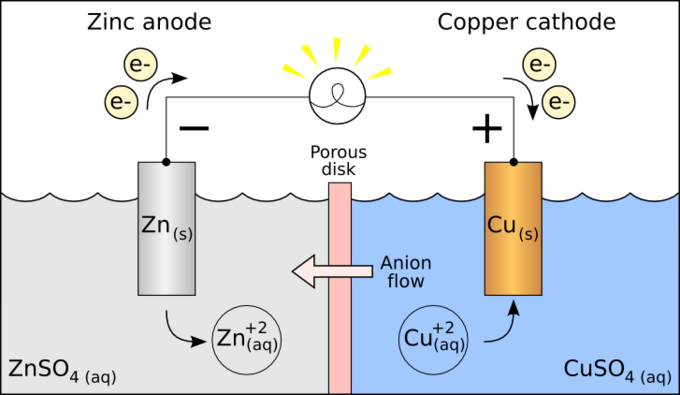
Cell Potentials Boundless Chemistry
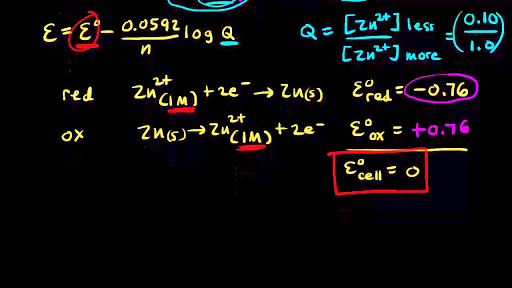
Redox Reactions And Electrochemistry Chemistry Library Khan Academy

Beaker Filled With Solution One Low Solute Concentration High Water Concentration And High Water Potential V Biology Revision Science Revision Cell Membrane

Cell Potentials Boundless Chemistry

Pin By Meionaise On Studyblr School Organization Notes Study Notes School Study Tips

Comments
Post a Comment